Looking Good Info About How To Draw Lewis Symbols

Locate these elements in the periodic table, and draw a lewis dot symbol that represents the number of valence electrons for an atom of each element.
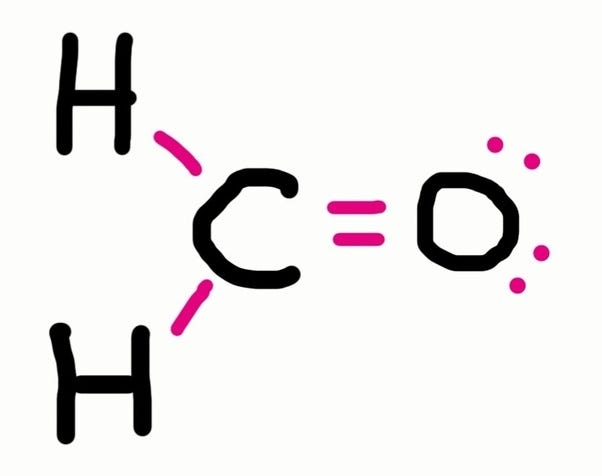
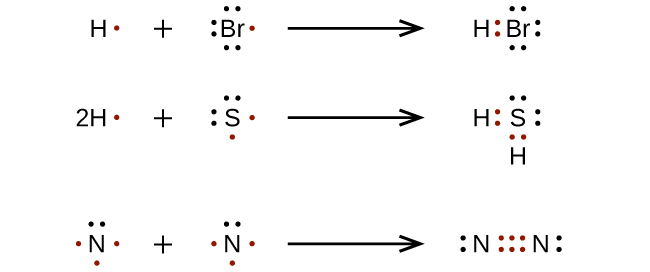
How to draw lewis symbols. Find the total valence electrons for the molecule. Stages to articulate the electron dot formula are stated beneath. A lewis electron dot formula comprises one dot for every valence electron and an element’s symbol.
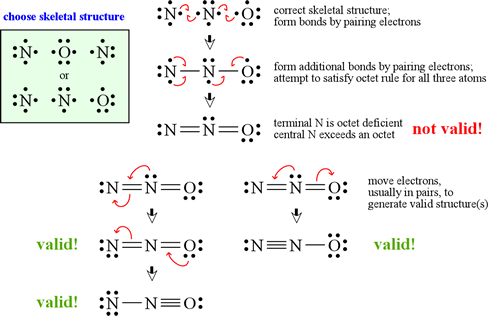
Put the least electronegative atom in the center. Causey explains valence electrons and how to use the periodic table to determine the valence electrons. The steps to draw the lewis structures of various types of compounds are given below:
Write the lewis symbols for the monatomic ions formed from the following elements: Transfer the electrons from metal to nonmetal. Hydrogen (h) always goes outside.
We can draw the lewis structure of any covalent molecule by following the six steps discussed earlier. In this case, we can condense the last few steps, since not all of them apply. Drawing lewis dot symbols or electron dot diagrams is an important skill in understanding molecular geometry and ionic crystals.
Valence electrons and lewis dot symbols. (1 \ (×\) 3) + (2 \ (×\) 4) + (1 \ (×\) 3) = 14. (5 \ (×\) 1) + (3 \ (×\) 1) = 8.
Then, determine whether the atoms are held together by a single, double, or. Write the lewis symbols for each of the following ions: [latex]\ce{cl}[/latex] [latex]\ce{na}[/latex] [latex]\ce{mg}[/latex] write lewis structures for the.






/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)